Introduction: It has been shown that leukemia-associated mutations can be detected in the blood of healthy individuals and their clonal expansion in hematopoietic stem and progenitor cell (HSPC) populations is referred to as Clonal Hematopoiesis (CH). In one study, a population analysis of a cohort of 17,182 patients performed by Bick et al. [PMID7944936] selected without prior screening for hematological malignancies, indicated that advancing age is directly associated with an increase in CH. When the mutant clone accounts for 4% of the nucleated blood cells, it is then referred to as Clonal Hematopoiesis of Indeterminant Potential (CHIP). CHIP has been classified by the World Health Organization as a premalignant state and risk criteria for neoplastic development. CHIP is highly associated with adverse outcomes including auto-immune or auto-inflammatory conditions, cardiovascular disease, cytopenia, cancers in general, and other hematological neoplasms. The associated hematopoietic neoplasms include myelodysplastic syndromes (MDS), myeloproliferative disorders (MPD), and acute myeloid leukemia (AML). Population studies reveal splicing factor mutations to be among the most common mutations in CHIP and are highly correlated with age and show a high hazard ratio for risk of developing secondary neoplasms including AML. RNA splicing factors U2AF1, SRSF2, and SF3B1 make up core components of the spliceosome and are critical to prevent aberrant splicing. Mutations in these factors have widespread effects implicating a multitude of cellular processes. There are several established mutant splicing factor mouse models that can be utilized; however, many employ non-specific or leaky Cre drivers.
Methods: To avoid non-specific and leaky Cre activation during aging, we used a Cre/lox-mediated U2af1(Q157R) conditional knock-in mouse model that does not contain an internal Cre driver. This mouse has a floxed wild-type U2af1 cDNA insert that expresses wild-type U2AF1 under normal circumstances. However, upon the exogenous addition of recombinant Cre the U2af1 cDNA is excised and the U2af1(Q157R) splicing factor mutation in exon 6 is expressed instead. This mouse model also possesses lox-stop-lox-YFP, leading to expression of YFP along with mutant U2AF1(Q157R) when Cre is introduced. We then isolated bone marrow from 6 week (young) and 6 months (aged) floxed Q157R/YFP mice and treated lineage-depleted bone marrow with recombinant Cre protein for two hours, followed by transplantation into mildly conditioned 6 week (young) and 18 month (old) wild-type mice. The percentage of YFP in the peripheral blood is quantified every 3 weeks. Full analyses, including RNA-seq, of the bone marrow progenitor populations after 3 months is in progress.
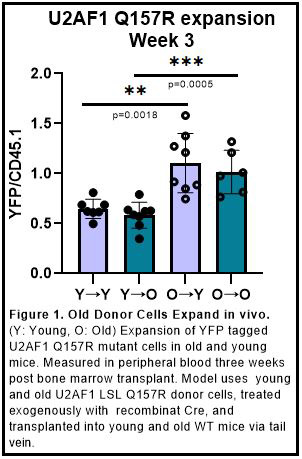
Results and Discussion: Using the previously mentioned bone marrow transplant model our work shows that when a U2AF1 mutation is introduced into young and old recipient mice, there is significant preferential outgrowth when the donor mice are aged (Figure 1). This is indicated by an increase in the ratio of YFP, which represents the U2AF1(Q157R) mutant, to CD45.1 events, which represent an internal engraftment control. This would suggest that the expansion of U2AF1(Q157R) splicing mutant cells seen in clinical data may be the result of intrinsic factors from the aging cell population. Comprehensive analysis of the effects of alternative splicing in this model is ongoing, however, these data provide support for further investigation of the intrinsic effects of splicing factor mutations in an aged context.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal